Open Heart Surgery
Open Heart Surgery
Open-heart surgery (OHS), also known as traditional heart surgery with a sternotomy, requires an incision in your chest to expose the heart. OHS for aortic valve replacement is a proven method that has been successfully performed since the 1960s.1
- Surgical aortic valve replacement with open heart surgery is proven to have better outcomes for patients who are overall healthy and strong enough to recover from this type of surgery.2,3
When considering open heart surgery for aortic valve replacement, you have several options including the Ross Procedure, a mechanical valve and a tissue valve.

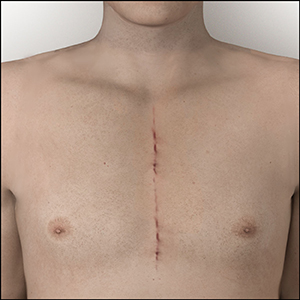
Ross Procedure
 |
Visit www.TheRossProcedure.org to learn more about the Ross Procedure. |
 |
Mechanical Valve
 |
Visit www.HeartValveChoice.com to learn more about the On-X Aortic Valve. * After 3 months standard therapy. See On-X Prosthetic Heart Valve Instructions for Use, https://www.onxlti.com/ifu/hv/, Accessed 12-04-2019. |
 |
Tissue Valve
 |
|
 |
Minimally Invasive Surgery
Minimally Invasive Surgical Aortic Valve Replacement
Minimally invasive surgical aortic valve replacement uses a smaller incision than a traditional open-heart surgery and has the potential to shorten hospital stays, quicken recovery and rehabilitation, and has better cosmetic results.12 Minimally invasive aortic valve replacement can be done using a mechanical valve or a tissue valve, but it is important to note that this surgery should be performed by a surgeon familiar with this technique. This smaller incision can be done through several approaches, two that are commonly used are shown below.
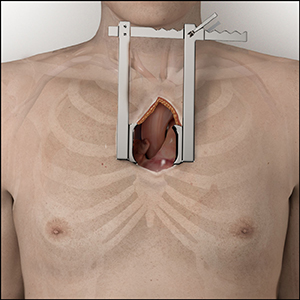
Mini or Hemi-Sternotomy
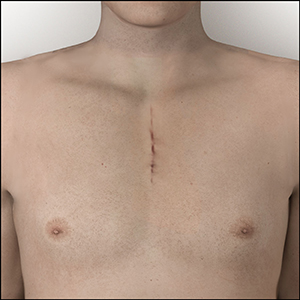
Right Thoracotomy
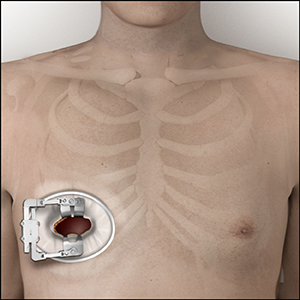
Mechanical Valve
|
Tissue Valve
|
TAVR
Transcatheter Aortic Valve Replacement (TAVR)

Transcatheter Aortic Valve Replacement (TAVR) is a relatively new technology typically performed in patients over the age of 75 to avoid the risk of surgery.13 In this procedure, the old, diseased aortic valve is not removed but rather a new tissue valve is inserted inside of the diseased aortic valve using a large catheter. A TAVR procedure can be done through either a transfemoral or a transapical approach.
There is little long-term published TAVR data on survival (life expectancy) or durability and there continues to be various generations of TAVR devices as this technology continues to evolve.
Click the years below to learn about the evolution of TAVR.

The first TAVR procedure
is performed.14

TAVR introduced in daily practice for inoperable patients at high surgical risk. Mean Age = 80 years old.15

TAVR indications expand to patients at intermediate surgical risk. Mean Age = 81 years old.13,16-17

TAVR expanded to low surgical risk patients. Mean Age = 75 years old.13,18-19
LOW RISK ≠ YOUNG PATIENTS

Will the value of TAVR vs. open heart surgery outweigh the unknown long-term outcomes and durability concerns?
Valve-in-Valve
Valve-in-Valve Procedure
Tissue valves, also called bioprosthetic valves, have the downside of a limited lifespan, or limited durability. These valves begin to breakdown and deteriorate, developing a condition called Structural Valve Deterioration (SVD).
There is no known medical therapy to prevent or treat SVD, so over time a tissue valve is likely to need replacing with another valve operation.8 This second valve operation can be done through either open-heart surgery, minimally invasive surgery, or a Valve-in-Valve (ViV) procedure.
A Valve-in-Valve (ViV) procedure does not remove the failed, diseased valve but rather inserts a second transcatheter valve inside the previous failed tissue valve.
Click on the steps below to see the process leading up to a Valve-in-Valve procedure.

Open Heart Surgery
Open Heart Surgery
Open-heart surgery (OHS), also known as traditional heart surgery with a sternotomy, requires an incision in your chest to expose the heart. OHS for aortic valve replacement is a proven method that has been successfully performed since the 1960s.1
- Surgical aortic valve replacement with open heart surgery is proven to have better outcomes for patients who are overall healthy and strong enough to recover from this type of surgery.2,3
When considering open heart surgery for aortic valve replacement, you have several options including the Ross Procedure, a mechanical valve and a tissue valve.


Ross Procedure

- A surgical operation that uses a patient’s own healthy and living pulmonary valve, called a pulmonary autograft, to replace the damaged aortic valve.
- This is the only aortic valve replacement option proven to result in life expectancy and quality of life as if they did not have valve surgery.4,5
- It is important to note that this procedure should be performed by a surgeon familiar with this technique.
Visit www.TheRossProcedure.org to learn more about the Ross Procedure.
Mechanical Valve

- Long-lasting valves made of durable materials that do not undergo Structural Valve Deterioration (SVD)
- Most mechanical heart valves are bileaflet, meaning that they have two “leaflets” to control the blood flow to a single direction.6
- While mechanical valves are often more durable, the risk for blood clot formation is higher, so lifelong use of blood thinner medication is necessary.
- The On-X Aortic Valve is in a category of its own as the only mechanical valve made of pure pyrolytic carbon designed to last a patient’s lifetime and approved to be used safely with less blood thinner compared to other mechanical valves.*,7
- This lower blood thinner requirement results in a significantly lower risk for bleeding by >60%.8
Visit www.HeartValveChoice.com to learn more about the On-X Aortic Valve.
* After 3 months standard therapy. See On-X Prosthetic Heart Valve Instructions for Use, https://www.onxlti.com/ifu/hv/, Accessed 12-04-2019.
Tissue Valve

- Also called “bioprosthetic valves”, tissue valves are made with animal tissue, typically pig or cow. Some tissue valves are mounted on a frame or stent, while others are stentless.
- Tissue valves typically do not require lifelong blood thinners.
- The downside to tissue valves is limited durability. Over time, these valves will begin to break down, leak, or become stiff and too small again, reintroducing symptoms of valve stenosis and regurgitation with a condition called Structural Valve Deterioration (SVD).
- Only tissue valves develop SVD and there is no known medical therapy to prevent or treat SVD.7
- SVD causes patients to feel tired and short of breath as their new aortic tissue valve deteriorates.9-11
- Implanted tissue valves that develop SVD may require reintervention, and the younger a patient is when they get a tissue valve, the faster they will experience the onset of SVD.7,9
Minimally Invasive Surgery
Minimally Invasive Surgical Aortic Valve Replacement
Minimally invasive surgical aortic valve replacement uses a smaller incision than a traditional open-heart surgery and has the potential to shorten hospital stays, quicken recovery and rehabilitation, and has better cosmetic results.12 Minimally invasive aortic valve replacement can be done using a mechanical valve or a tissue valve, but it is important to note that this surgery should be performed by a surgeon familiar with this technique. This smaller incision can be done through several approaches, two that are commonly used are shown below.
Mini or Hemi-Sternotomy


Right Thoracotomy


Mechanical Valve
- Long-lasting valves made of durable materials that do not under go Structural Valve Deterioration (SVD)
- Most mechanical heart valves are bileaflet, meaning that they have two “leaflets” to control the blood flow to a single direction.6
- While mechanical valves are often more durable, the risk for blood clot formation is higher, so lifelong use of blood thinner medication is necessary.
Tissue Valve
- Also called “bioprosthetic valves”, tissue valves are made with animal tissue, typically pig or cow. Some tissue valves are mounted on a frame or stent, while others are stentless.
- Tissue valves typically do not require lifelong blood thinners.
- The downside to tissue valves is limited durability. Over time, these valves will begin to break down, leak, or become stiff and too small again, reintroducing symptoms of valve stenosis and regurgitation with a condition called Structural Valve Deterioration (SVD).
- Only tissue valves develop SVD and there is no known medical therapy to prevent or treat SVD.7
- SVD causes patients to feel tired and short of breath as their new aortic tissue valve deteriorates.9-11
- Implanted tissue valves that develop SVD may require reintervention, and the younger a patient is when they get a tissue valve, the faster they will experience the onset of SVD.7,9
TAVR
Transcatheter Aortic Valve Replacement (TAVR)
 Transcatheter Aortic Valve Replacement (TAVR) is a relatively new technology typically performed in patients over the age of 75 to avoid the risk of surgery.13 In this procedure, the old, diseased aortic valve is not removed but rather a new tissue valve is inserted inside of the diseased aortic valve using a large catheter. A TAVR procedure can be done through either a transfemoral or a transapical approach.
Transcatheter Aortic Valve Replacement (TAVR) is a relatively new technology typically performed in patients over the age of 75 to avoid the risk of surgery.13 In this procedure, the old, diseased aortic valve is not removed but rather a new tissue valve is inserted inside of the diseased aortic valve using a large catheter. A TAVR procedure can be done through either a transfemoral or a transapical approach.
There is little long-term published TAVR data on survival (life expectancy) or durability and there continues to be various generations of TAVR devices as this technology continues to evolve.
Click the years below to learn about the evolution of TAVR.
2002
The first TAVR procedure
is performed.14
2007
TAVR introduced in daily practice for inoperable patients at high surgical risk. Mean Age = 80 years old.15
2016
TAVR indications expand to patients at intermediate surgical risk. Mean Age = 81
years old.13,16-17
2019
TAVR expanded to low surgical risk patients. Mean Age = 75 years old.13,18-19
LOW RISK ≠ YOUNG PATIENTS
FUTURE
OF
TAVR
Will the value of TAVR vs. open heart surgery outweigh the unknown long-term outcomes and durability concerns?
Valve-in-Valve
Valve-in-Valve Procedure
Tissue valves, also called bioprosthetic valves, have the downside of a limited lifespan, or limited durability. These valves begin to breakdown and deteriorate, developing a condition called Structural Valve Deterioration (SVD).
There is no known medical therapy to prevent or treat SVD, so over time a tissue valve is likely to need replacing with another valve operation.8 This second valve operation can be done through either open-heart surgery, minimally invasive surgery, or a Valve-in-Valve (ViV) procedure.
A Valve-in-Valve (ViV) procedure does not remove the failed, diseased valve but rather inserts a second transcatheter valve inside the previous failed tissue valve.
Click on the steps below to see the process leading up to a Valve-in-Valve procedure.
Patient’s own aortic valve becomes diseased and calcified, requiring replacement.
![bioprosthetic_1000x850-1 ProEXR File Description
=Attributes=
cameraAperture (float): 36
cameraFarClip (float): 3.40282e+038
cameraFarRange (float): 39370.1
cameraFov (float): 24.739
cameraNearClip (float): 0
cameraNearRange (float): 0
cameraProjection (int): 0
cameraTargetDistance (float): 538.19
cameraTransform (m44f): [{0.999955, -0.000516554, 0.00943813, 23.9083}, {0.00945225, 0.0546463, -0.998461, -345.946}, {-1.74623e-010, 0.998505, 0.0546488, 88.5385}, {0, 0, 0, 1}]
channels (chlist)
compression (compression): Zip16
dataWindow (box2i): [0, 0, 3299, 3961]
displayWindow (box2i): [0, 0, 3299, 3961]
gamma (float): 1
lineOrder (lineOrder): Increasing Y
pixelAspectRatio (float): 1
screenWindowCenter (v2f): [0, 0]
screenWindowWidth (float): 1
tiles (tiledesc): [64, 64]
type (string): "tiledimage"
=Channels=
A (half)
B (half)
G (half)
R (half)](https://heartvalveinsights.com/wp-content/uploads/2021/01/bioprosthetic_1000x850-1.jpg)
Patient has tissue valve implanted for surgical aortic valve replacement. This valve can start to fail after only 6 years.20
![Biovalve-calcification-side_1000x850 ProEXR File Description
=Attributes=
cameraAperture (float): 36
cameraFarClip (float): 3.40282e+038
cameraFarRange (float): 39370.1
cameraFov (float): 24.739
cameraNearClip (float): 0
cameraNearRange (float): 0
cameraProjection (int): 0
cameraTargetDistance (float): 538.19
cameraTransform (m44f): [{0.999955, -0.000516554, 0.00943813, 23.9083}, {0.00945225, 0.0546463, -0.998461, -345.946}, {-1.74623e-010, 0.998505, 0.0546488, 88.5385}, {0, 0, 0, 1}]
channels (chlist)
compression (compression): Zip16
dataWindow (box2i): [0, 0, 3299, 3961]
displayWindow (box2i): [0, 0, 3299, 3961]
gamma (float): 1
lineOrder (lineOrder): Increasing Y
pixelAspectRatio (float): 1
screenWindowCenter (v2f): [0, 0]
screenWindowWidth (float): 1
tiles (tiledesc): [64, 64]
type (string): "tiledimage"
=Channels=
A (half)
B (half)
G (half)
R (half)](https://heartvalveinsights.com/wp-content/uploads/2021/01/Biovalve-calcification-side_1000x850.jpg)
Tissue valve develops SVD and requires reoperation, or a ViV procedure, as early as
8 years after implant.21
![ViV_1000x850-1 ProEXR File Description
=Attributes=
cameraAperture (float): 36
cameraFarClip (float): 3.40282e+038
cameraFarRange (float): 39370.1
cameraFov (float): 24.739
cameraNearClip (float): 0
cameraNearRange (float): 0
cameraProjection (int): 0
cameraTargetDistance (float): 538.19
cameraTransform (m44f): [{0.999955, -0.000516554, 0.00943813, 23.9083}, {0.00945225, 0.0546463, -0.998461, -345.946}, {-1.74623e-010, 0.998505, 0.0546488, 88.5385}, {0, 0, 0, 1}]
channels (chlist)
compression (compression): Zip16
dataWindow (box2i): [0, 0, 3299, 3961]
displayWindow (box2i): [0, 0, 3299, 3961]
gamma (float): 1
lineOrder (lineOrder): Increasing Y
pixelAspectRatio (float): 1
screenWindowCenter (v2f): [0, 0]
screenWindowWidth (float): 1
tiles (tiledesc): [64, 64]
type (string): "tiledimage"
=Channels=
A (half)
B (half)
G (half)
R (half)](https://heartvalveinsights.com/wp-content/uploads/2021/01/ViV_1000x850-1.jpg)
Transcatheter valve is inserted inside the first failed tissue valve, this is a ViV procedure. What happens next since there is no long-term data on ViV?
References
- Harken DE, et al. J Thorac Cardiovasc Surg 1960;40:744–762.
- Otto CM, et al. Circulation. 2021;143: doi: 10.1161/CIR.0000000000000923.
- Kytö V, et al. Ann Thorac Surg 2019;110(1):102-10.
- Mazine A, et al. J Am Coll Cardiol 2018;72(22):2761-77.
- El Hamamsy I, et al. Lancet 2010;376(9740):524-31.
- Rajashekar P. J Pract Cardiovasc Sci 2015;1:289-93.
- Nishimura RA, et al. J Am Coll Cardiol 2017;70:252-89.
- Puskas JD, et al. J Am Coll Cardiol 2018;71:2717-26.
- Rodriguez-Gabella T, et al. J Am Coll Cardiol 2017;70(8):1013-28.
- "Aortic Stenosis", Mayo Clinic [Online]. Available: https://www.mayoclinic.org/diseases-conditions/aortic-stenosis/symptoms-causes/syc-20353139 [Accessed 01-04-2021].
- "Aortic Valve Regurgitation", Mayo Clinic [Online]. Available: https://www.mayoclinic.org/diseases-conditions/aortic-valve-regurgitation/symptoms-causes/syc-20353129 [Accessed 01-04-2021].
- Kaczmarczyk M, et al. Kardiochirurgia i Torakochirurgia Polska = Polish Journal of Cardio-thoracic Surgery 2015;12(2):103-110.
- Carroll JD, et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. Ann Thorac Surg. 2020; doi: 10.1016/j.athoracsur.2020.09.002.
- “Fifteen Years of TAVR; Where Are We Now?” American College of Cardiology, 20 Jul. 2017.
- Kappetein AP, et al. Eur Heart J. 2012;33:2403-18.
- Leon MB, et al. N Engl J Med. 2016;374:1609-20.
- Makkar RR, et al. N Eng J Med. 2020;382:799-809.
- Popma JJ, et al. N Engl J Med. 2019;380:1706-15.
- Mack MJ, et al. N Eng J Med. 2019;380:1695-1705.
- Bourguignon T, et al. Eur J Cardiothorac Surg. 2016;1462-8.
- Minakata K, et al. J Card Surg. 2015;30(5):405–13.
Important Safety Information
On-X® Prosthetic Heart Valve
Indications:
The On-X Prosthetic Heart Valve is indicated for the replacement of diseased, damaged, or malfunctioning native or prosthetic heart valves in the aortic and mitral positions.
Contraindications (Who should not use):
The On-X Prosthetic Heart Valve is contraindicated for patients unable to tolerate anticoagulation therapy (blood thinner medications).
The safety and effectiveness of the On-X Prosthetic Heart Valve has not been established for the following specific populations because it has not been studied in these populations:
- patients who are pregnant;
- nursing mothers;
- patients with chronic endocarditis;
- patients requiring pulmonary or tricuspid replacement.
Warnings:
For single use only. Surgeon should not use the On-X Prosthetic Heart Valve if:
- the prosthesis has been dropped, damaged, or mishandled in any way;
- the expiration date has elapsed;
- the tamper evident seal is broken;
- the serial number tag does not match the serial number on the container label.
Surgeon should not pass a catheter, surgical instrument, or transvenous pacing lead through the prosthesis as this may cause valvular insufficiency, leaflet damage, leaflet dislodgment, and/or catheter/instrument/lead entrapment.
Surgeon should not resterilize the On-X Prosthetic Heart Valve.
Precautions:
Surgeon should handle the prosthesis with only On-X Life Technologies, Inc. (On-XLTI) On-X Prosthetic Heart Valve Instruments. Only On-XLTI On-X Prosthetic Heart Valve sizers should be used during the selection of the valve size; other sizers may result in improper valve selection.
Surgeon should avoid contacting the carbon surfaces of the valve with gloved fingers or any metallic or abrasive instruments as they may cause damage to the valve surface not seen with the unaided eye that may lead to accelerated valve structural dysfunction, leaflet escape, or serve as a nidus for thrombus formation.
Surgeon should avoid damaging the prosthesis through the application of excessive force to the valve orifice or leaflets.
Potential Adverse Events:
Adverse events potentially associated with the use of prosthetic heart valves (in alphabetical order) include, but are not limited to:
- angina (chest pain)
- cardiac arrhythmia (irregular heart beat)
- endocarditis (infection within the heart)
- heart failure
- hemolysis (red blood cell damage)
- hemolytic anemia (disorder in which red blood cells are destroyed faster than they are made)
- hemorrhage (bleeding)
- myocardial infarction (heart attack)
- prosthesis leaflet entrapment (blockage of device leaflet)
- prosthesis nonstructural dysfunction (device leakage, blockage, inappropriate sizing, etc.)
- prosthesis pannus (excess tissue ingrowth on device)
- prosthesis perivalvular leak (leakage around the outside of the device)
- prosthesis regurgitation (blood flow backwards into device)
- prosthesis structural dysfunction (mechanical failure of device)
- prosthesis thrombosis (blood clot attached to or near device)
- stroke
- thromboembolism (blood vessel obstruction by a blood clot)
It is possible that these complications could lead to:
- reoperation
- explantation
- permanent disability
- death
Mechanical prosthetic heart valves produce audible sounds as a normal function of their operation. In some patients, these sounds may be objectionable.
010122 77 E (2020-12)
